BenevolentAI, a startup which has raised $292 million to apply AI to create drugs faster, today says it has uncovered an already approved drug as a potential treatment for COVID-19, after it applied its AI platform and team to the problem. The revelation, which has now appeared in peer-reviewed scientific journals and has already entered clinical trials with a major pharmaceutical company, could offer a glimmer of hope to a world locked-down by the pandemic.
In February, BenevolentAI set up a specialist scientific team and launched an investigation using its drug discovery platform.
Baroness Joanna Shields, CEO of BenevolentAI, explained: “In response to the COVID-19 global health emergency, we turned our AI drug discovery and development platform toward understanding the body’s response to this novel infectious disease.”
Key to their approach was that “rather than focusing solely on drugs that could affect the virus directly, we explored ways to inhibit the cellular processes that the virus uses to infect human cells,” she said.
The idea was to identify approved drugs that could potentially stop the progression of COVID-19, inhibit the “cytokine storm” and reduce the inflammatory damage associated with this disease.
Diseases such as covid-19 and influenza can be fatal due to an overreaction of the body’s immune system called a cytokine storm.
Cytokines are small proteins released by many different cells in the body, including those of the immune system where they coordinate the body’s response against infection and trigger inflammation.
The body’s response to infection goes into overdrive when SARS-CoV-2 – the virus behind the COVID-19 pandemic – enters the lungs, triggering an immune response, and attracting immune cells to the region to attack the virus. This resulting in localized inflammation. Some people experience worse symptoms than others at this point. But in some people, excessive or uncontrolled levels of cytokines are released which then activate more immune cells, resulting in ‘hyperinflammation’ which can seriously harm or even kill the person.
BenevolentAI’s team fed everything they knew about COVID-19, and the drugs that could inhibit the cellular processes that the virus uses, into their platform.
In an interview with TechCrunch, Peter Richardson, BenevolentAI’s VP of Pharmacology, explained how the discovery came about.
“Using the BenevolentAI Knowledge Graph there were two processes. One was finding the connections, and the regulators,” he said.
“It’s incredibly, incredibly difficult to hold in your head what’s irrelevant, all the time, without having the Knowledge Graph to show you the basic points. It’s really good at showing the basic interactions that are so important in understanding a biological process. Cellular tracking is an amazingly complicated thing to process.”
But, he said, the BenevolentAI platform handled the information with aplomb: “This took an hour for the platform to process.”
The next process was for the human team to find possible drugs to inhibit regulators. These were then fed into the Knowledge Graph. Richardson said this “took roughly half an hour to process.”
The result was that they identified baricitinib as a potential drug with both anti-viral and anti-cytokine properties, with 90 minutes of computing time, inside three days of additional human work.
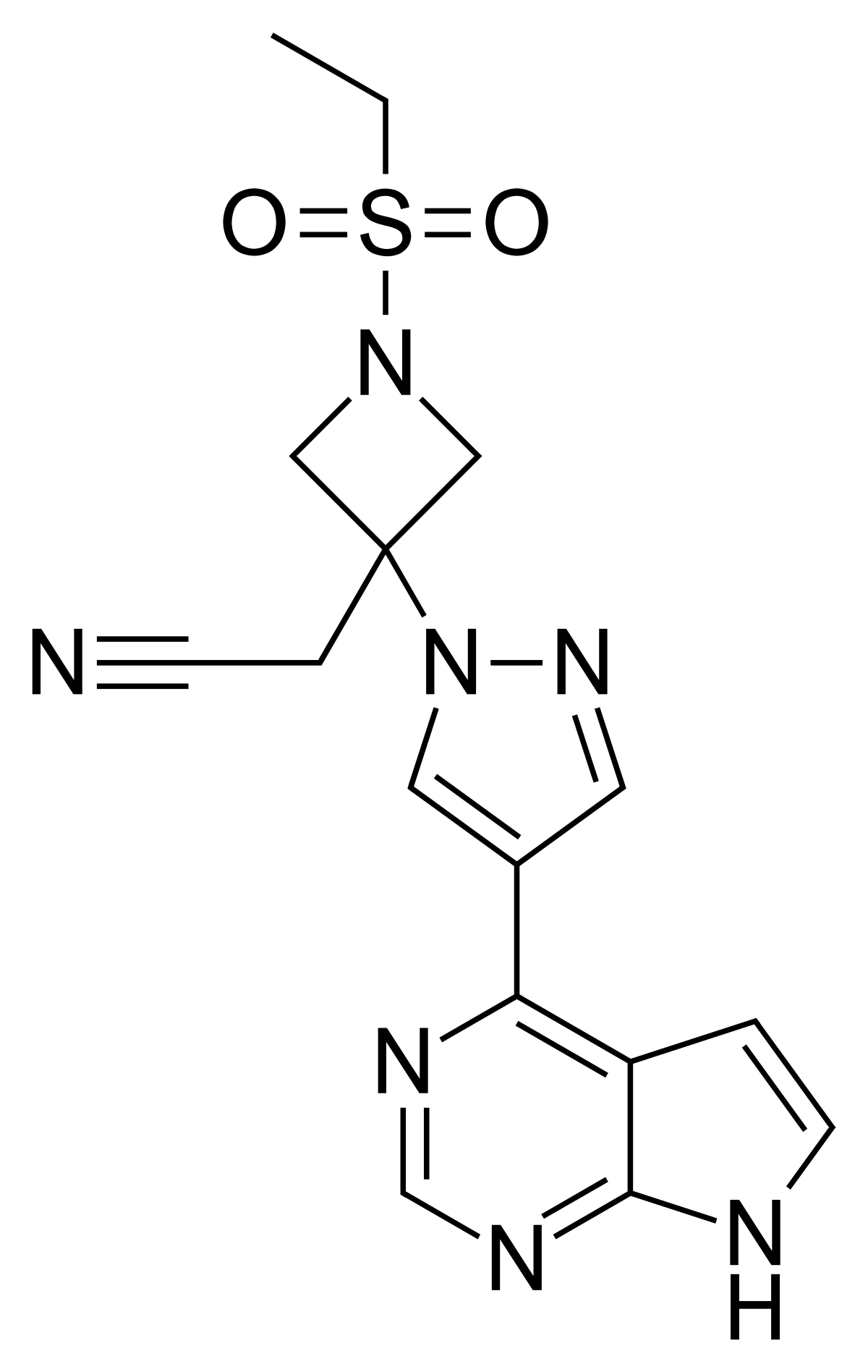
Benevolent’s research findings were published in The Lancet in early February and again twice in the Lancet Infectious Diseases journal. These proposed baricitinib as a potential treatment with both anti-viral and anti-inflammatory properties for COVID-19 patients admitted to hospital prior to the development of critical lung damage.
By March, investigator-led studies began recruiting and treating infected patients with baricitinib. Today, Eli Lilly and the US National Institute for Allergies and Infectious Diseases (NIAID) have announced that the drug will now begin it’s first large randomized trial in COVID-19 patients.
Baricitinib, sold as a prescription drug called Olumiant, is an already approved drug developed by Eli Lilly and Incyte for the treatment of rheumatoid arthritis.
The randomized trial announced by Eli Lilly with NIAID will investigate the efficacy and safety of baricitinib as a potential treatment for patients with serious COVID-19 infections.
The study will begin in the US in late April with planned expansion to additional sites in Europe and Asia, with the results being expected within the next two months. This new trial joins a Canadian government randomised trial already underway assessing baricitinib as a potential treatment.
Commenting, Shields said: “We are pleased that Eli Lilly is progressing baricitinib to clinical testing for COVID-19 patients. While we wait for a vaccine to be developed, there is an immediate need for medicines that can prevent life-threatening respiratory and other serious complications of COVID-19 infections.”
Daniel Skovronsky, M.D., Ph.D., Lilly’s chief scientific officer and president of Lilly Research Laboratories said: “Lilly is moving at top speed and using all available resources to help fight this pandemic. Developing potential therapeutic medicines for COVID-19 is part of our vital and humanitarian mission.”
Professor Justin Stebbing from Imperial College, London, who has been collaborating on this work between Eli Lilly and BenevolentAI, also commented, saying: “There are no specific therapeutic agents for any coronavirus infections – we rely on quarantine, isolation and public health policies to prevent disease spread, and on supportive care measures for those who become ill. What we lack is a specific agent to treat the infected and, optimally, decrease viral shedding and subsequent transmission. The results of such trials will be central to clinical care as the outbreak continues and we anticipate that this treatment will improve mortality and reduce the pressure on hospitals and ICU’s worldwide. This research is notable for its incredible speed from computer to bench and bedside within a few months.”
Commenting, Richardson added: “If you turned the BenevolentAI 250-person team and turned all of them into 65-year old ex-pharmacology teachers, it would have taken probably a year to come up with this treatment. Instead, it took my three colleagues working so two hours a day, and myself working full time, three days to come up with this. We’ve gone from computer to bedside, as it were, in two months.”