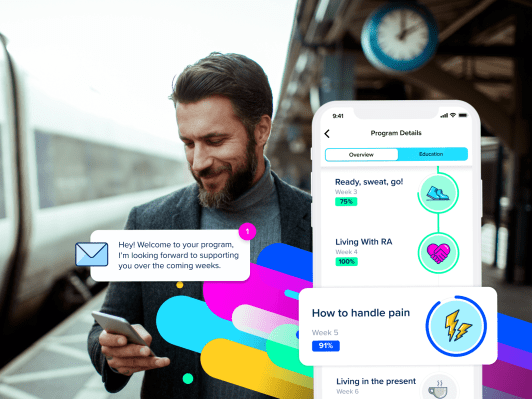
Digital therapeutics plus pills? Iceland’s Sidekick Health has developed a gamified digital care platform that’s designed to support healthcare outcomes by applying personalized behavioral lifestyle nudges, including alongside clinical treatments like drugs, to augment, extend and support patient care for a range of chronic diseases and conditions from cancer to cardiovascular health, diabetes and arthritis — a formula that’s now scored it $55M in Series B growth funding.
The new round is led by London-based VC firm Novator Ventures, with participation from Wellington Partners, Asabys Partners, and Frumtak Ventures, as well as a US-based strategic investor which it’s not disclosing yet but says will be revealed at a later stage. The 2014-founded startup raised a $20M Series A back in 2020 from many of the same investors.
As the Series B closes, Novator Ventures’ general partner and founder Birgir Mar Ragnarsson is joining Sidekick’s board.
Commenting in a statement, he said: “It has been impressive to follow the rapid growth of the company from the close of its A round 18 months ago. The company may have begun in the Nordics but I am proud to say that Sidekick is now a globally-recognized digital therapeutics player. Novator Ventures recognizes the immense opportunities presented by third generation therapeutics and Sidekick’s ability to scale and operate at the global level. We look forward to working closely with the Sidekick team to transform how healthcare is delivered.”
Sidekick Health still isn’t breaking out overall customer numbers (it’s a b2b digital health business so its targeting health insurance firms and pharma companies) but says it’s inked partnerships with some of the biggest names in healthcare — such as US-based Anthem to offer “digital-first” care programs, and global pharma giants Bayer and Pfizer, to develop what it describes as “integrated combination therapeutics consisting of a molecular drug and a digital therapeutic”.
The startup tells us its platform has helped 40,000+ patients globally at this stage, with its products currently available in six languages. It largest markets are Europe and the U.S., while it has offices in the U.S., Germany, Sweden and its home market of Iceland. Flush with growth funding it says it has a big U.S. push planned.
“Europe and the U.S. are currently our largest markets but we are forging some partnerships in Asia,” it tells TechCrunch. “But it is early days, and we will be focusing hard on increasing our commercial footprint in the U.S. going forward.
“Sidekick will use the funding to support and expand our existing U.S. presence. Our North American strength and focus is exemplified by the recent additions of Pamela Stahl (CCO and President, North America) and Mitchell Mudra (COO) to the team. By 2026, it is estimated a billion people will be served by some form of DTx [digital therapeutics] annually. Coupled with the U.S. spending a very high proportion of GDP on healthcare, the U.S. is a market where our products and services will achieve the strongest patient outcomes.”
The startup tells TechCrunch it grew revenue threefold in 2021 — attributing that growth to a mix of existing and new commercial relationships. “This year Sidekick plans to double our team from 120 to 240 team members across our four office locations,” it adds.
Pharma giants look keen on digital therapeutics not only as a scalable tool to (potentially) augment the efficacy of their drugs with app-based support (e.g. by putting digital tools in patients’ hands that can help remind them to take pills and support them in other ways, such as to make beneficial lifestyle changes around diet and exercise, or get on-demand help with mental health issues or pain management etc), but as a way to extend the value of medicines — enabling drug giants to file new patents linking existing medicines to digital therapeutic programs that are far cheaper and easier to develop and iterate than he costly business of drug discovery and research.
“We are building towards a portfolio of over 40 medical-grade digital therapeutics by 2026,” says Sidekick, discussing its product roadmap. “Currently, 18 are in Research & Development, with a total of 14 commercial partnerships secured so far, either with payers or pharma partners or, in some cases, both.”
“Sidekick has commercialized digital therapeutic products already, in therapeutic areas ranging from rheumatoid arthritis, ulcerative colitis to nonalcoholic steatohepatitis and breast cancer,” it adds. “We will prioritize a substantial amount of our resources towards expanding our oncology portfolio, as well as investing into increased personalization, which will allow us to serve people with multiple chronic conditions even better.”
With Series B funding in the bank, Sidekick also has more partnerships in the works — with three new collaborations set to be announced in the coming months including one focused on supporting patients with breast cancer by helping patients manage side effects.
The startup has published a number of studies aimed at proving out the efficacy of its digital therapeutics –including looking at use of its platform to reduce stress and fatigue in patients with inflammatory bowel disease; or this small feasibility study into improving disease management for patients with ulcerative colitis; and this small randomized control trial examining use of its digital lifestyle program for outpatient treatment of Type 2 diabetes, to name a few — and it says it takes “life science-grade evidence generation very seriously to ensure that we offer clinical companies a clinical-grade product for their patients”.
“Top 10 and 20 pharma and Top 3 Payers are partnering with us on their ‘future of franchise’ assets, in areas like oncology, metabolic, cardiovascular, and immunology and immunotoxicology,” it adds, suggesting that its “life science quality evidence rigor and approach to precision medicine is driving this”.